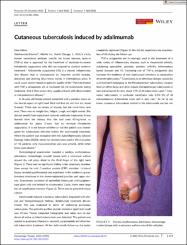
Cutaneous tuberculosis induced by adalimumab
View/
Access
info:eu-repo/semantics/embargoedAccessDate
07.04.2022Metadata
Show full item recordCitation
Gürel, G., Özdemir, Ç., & Durusu, İ. N. (2022). Cutaneous tuberculosis induced by adalimumab. Dermatologic Therapy, e15503-e15503.Abstract
Dear Editor,
Adalimumab (Humira®, AbbVie Inc., North Chicago, IL, USA) is a fully human monoclonal antibody specific for tumor necrosis factor-α (TNF-α) that is approved for the treatment of moderate-to-severe hidradenitis suppurativa who did not respond to classical systemic treatment.1 Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that is characterized by recurrent painful nodules, abscesses and draining sinus tracts mainly in intertriginous areas. It could cause severe impact on patients' quality of life.2 Patients treated with TNF-α antagonists are at increased risk for tuberculosis during treatment. And if that occurs they usually present with disseminated or extrapulmonary disease.















